
Urine-blood PCO 25.5, oral furosemide is administered at 1 mg/kg and fludrocortisone at 0.025 mg/kg.
The urine-blood PCO 2 > 20 mmHg is seen with normal acid secretion.

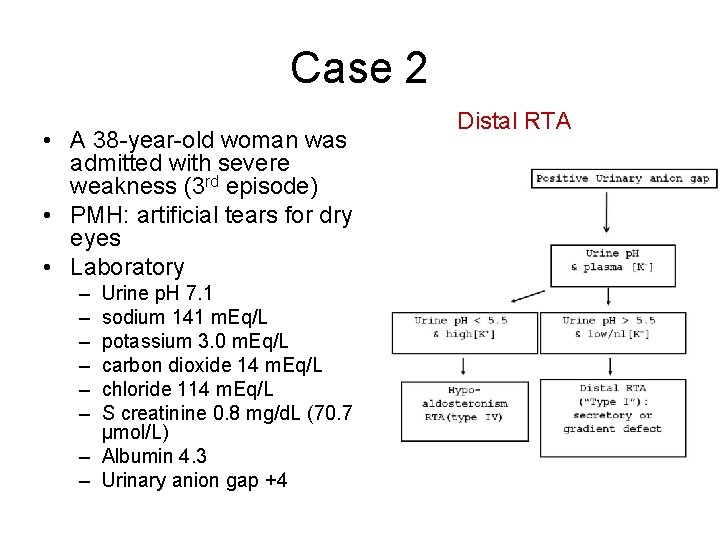
At a urine pH>7.5 and serum bicarbonate 23–25 meq/L, the urine PCO 2 should be >70 mmHg. In the absence of CA II, this slowly gets converted to CO 2 which remains trapped in the lumen. Urine-blood PCO 2: After a load of sodium bicarbonate, the secreted H + reacts with the luminal HCO 3 - in the distal lumen forming carbonic acid. The normal fractional excretion of bicarbonate is 15% suggests bicarbonate wasting The fractional excretion of bicarbonate is calculated The total plasma CO 2 should fall by 3–5 meq/L and urine pH should be 7.5. Ammonium chloride is given at 0.1 mg/kg followed by measurement of urine pH every hour for the next 2–8 h. If systemic acidosis is mild, it can be induced using the ammonium chloride loading test. Urine pH: A urine pH >5.5 in the presence of metabolic acidosis is suggestive of distal acidification defect seen with dRTA. Urine analysis: A routine analysis of urine for the presence of proteinuria (usually non-nephrotic range, i.e., urine albumin 1+–2+), glycosuria indicates the presence of generalized tubular wasting seen with Fanconi syndrome Once, the diagnosis of RTA is confirmed, the next step is to differentiate between the different types of RTA Urinary ammonium excretion is estimated to be half the urinary osmolal gap and is considered to be increased if it >100 mosm/kg. Urine osmolal gap = measured osmolality – calculated osmolality.Ĭalculated urine osmolality= 2 + urea + glucose 6 18 Urine osmolal gap: This is used in situations where the urine anion gap is not reliable. Proximal RTA is caused by an impairment of bicarbonate reabsorption with intact distal acidification mechanisms and is characterized by decreased renal bicarbonate threshold (14–18 mEq/L), defect in ammonia generation, and ability to lower urine pH 6.5, bicarbonate is excreted, hence, urinary anion gap does not reflect urinary ammonium levels. RTA: Renal tubular acidosis, CNS: Central nervous system Type II RTA – proximal RTA Phosphatidylinositol 4,5-bisphosphate 5-phosphataseĪTPase copper transporting beta-polypeptide Galactose-1-phosphate uridylyltransferase Medications – Amphotericin, lithium, and aminoglycosides. EtiologyĪutoimmune – Sjogren’s syndrome, systemic lupus erythematosus, and Graves’ disease Hypercalciuria and hypocitraturia are seen in dRTA which make the children prone for early-onset nephrocalcinosis and nephrolithiasis. It is more commonly associated with dRTA as potassium is the only available cation for exchange with sodium and distal secretion of H + is impaired. Hypokalemia seen in RTA is due to proximal and distal wasting of sodium, leading to volume contraction and secondary hyperaldosteronism. Extracellular bicarbonate and hydroxyapatite in the bones serve as buffers to neutralize the accumulated acid. Failure of the distal tubules to excrete the acid load generated by metabolism and in the growing bone in children leads to accumulation of acid and worsening base deficit. The hallmark of distal RTA is the inability to lower urine pH maximally in the presence of moderate-to-severe metabolic acidosis.

Įxport to PPT TYPES OF RTA Type 1 RTA – distal RTA (dRTA)ĭRTA, characterized by impaired hydrogen ion secretion in the distal tubules, is commonly seen due to inherited mutations of transporters in the distal tubule. Bicarbonate ions from the dissociated carbonic acid exit through the basolateral membrane by the sodium bicarbonate exchanger (NBCE1). The carbon dioxide generated diffuses freely into the proximal tubule cell and reacts with water to form carbonic acid, a reaction catalyzed by carbonic anhydrase (CA II). In the lumen, the secreted hydrogen ions combine with HCO 3 - to form carbonic acid which rapidly dissociates into carbon dioxide and water, a reaction catalyzed by carbonic anhydrase. The proximal tubule is responsible for reabsorption of 85–90% of filtered bicarbonate through secretion of protons (H +) through the sodium hydrogen exchangers and proton pumps (H +ATPase). ACID–BASE HOMEOSTASIS: ROLE OF THE KIDNEY Bicarbonate reabsorption in the proximal tubule Renal tubular acidosis (RTA) arises from the kidney’s inability to excrete enough acid or retain enough bicarbonate, in the presence of normal renal function, resulting in a clinical syndrome characterized by persistent normal anion gap hyperchloremic metabolic acidosis.

The kidneys play a key role in preserving the acid–base homeostasis in the body by excretion of acid and regeneration of bicarbonate.


 0 kommentar(er)
0 kommentar(er)
